PiLR Health Product Features
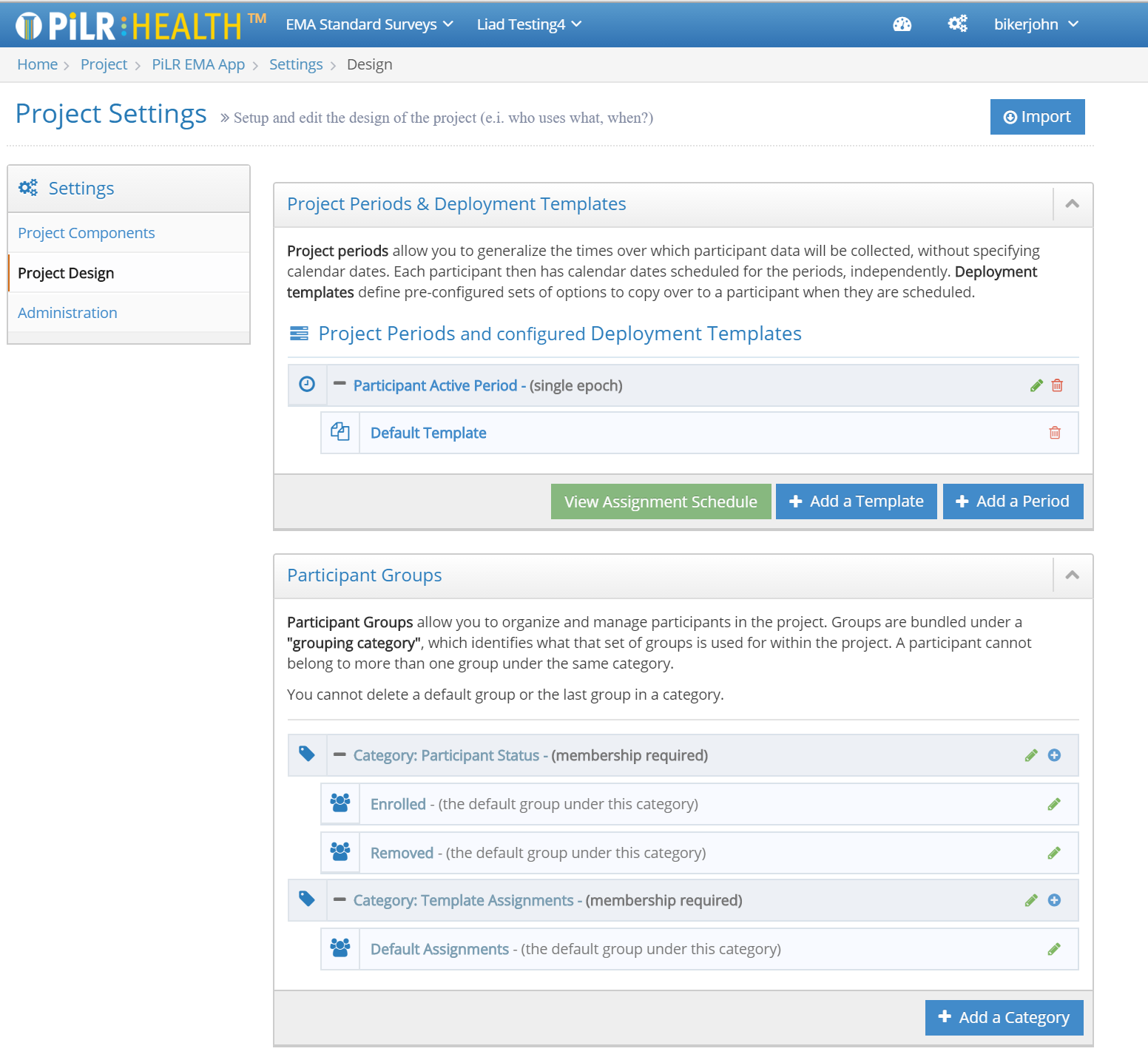
Tools for Configuring Studies
PiLR Health provides a rich set of tools for configuring studies.
- PiLR Health was designed by researchers for researchers, so the system can accomodate real world study designs (like a cross-over study, or a dual cohort control and treatment design)
- Utilize mobile sensors and smartphones to gather information from study participants while staying within IRB guidelines for privacy and security
- Develop and automate sophisticated study data collection protocols that can be managed by the system which reduces researcher workloads during the active data collection period
- Manage research team member priveledges
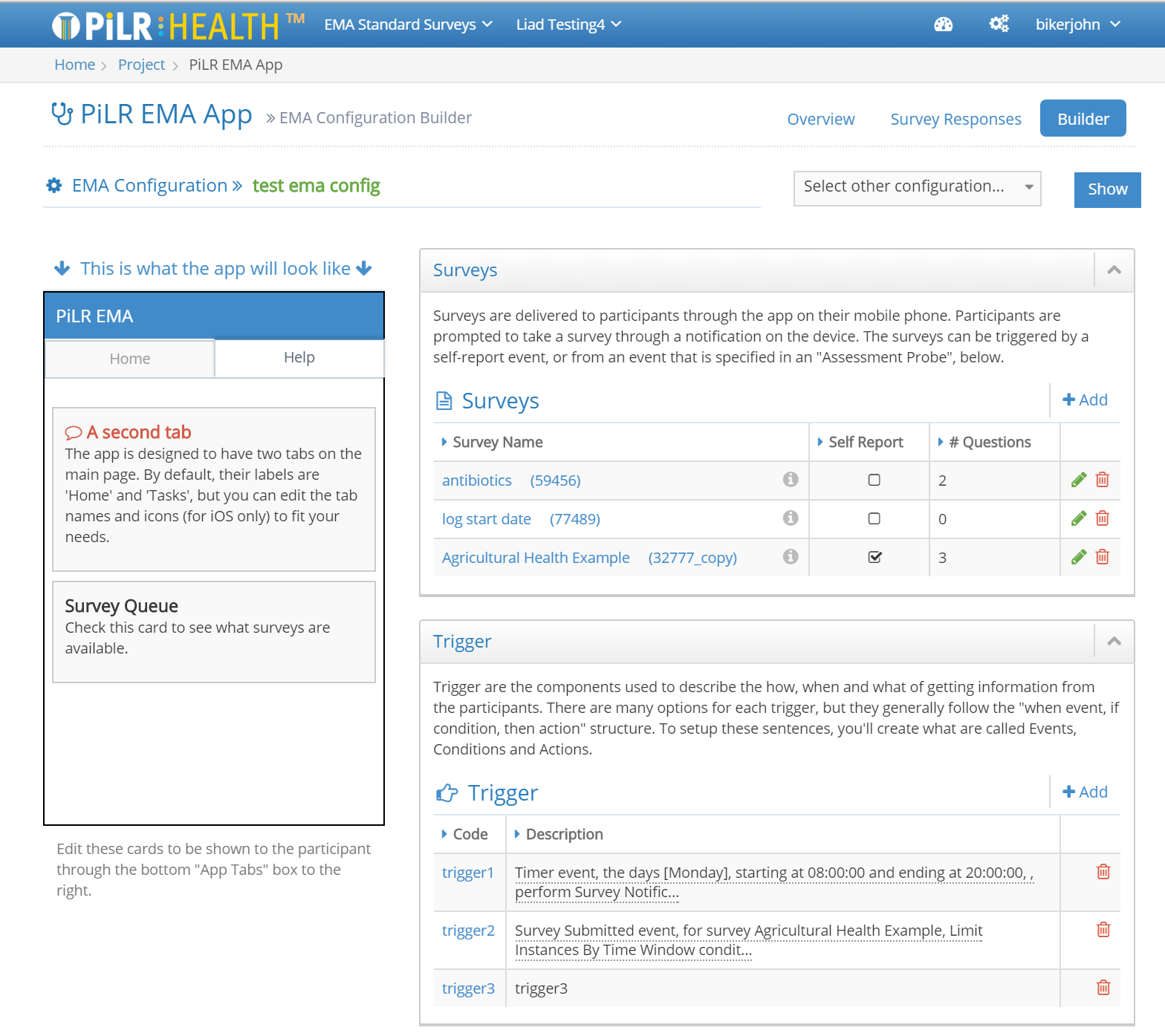
Tools for Creating Custom Study Content
The PiLR Health EMA Builder page makes it possible for research teams to:
- Create custom content to be delivered to study participants
- Participants can receive instructions about what to do, including links to web sites or media
- Study teams can create surveys to collect subjective information from participants
- Surveys can include media types like photographs and sound
- It is possible to change participant navigation through surveys by using branching rules
- Survey questions can made required
- More than 1 survey can be active at a time
- PiLR Health uses triggers to control when surveys are offered to participants
- Surveys can be initiated based on time and date, participant location, participant activity level, and completion of a previous activity
- Conditions can be applied to these events making it possible to control the number of times an event can occur within a day, or a custom time period
- Thresholds can be set for an activity — an example would be presenting a survey if a participant was walking for 75% of any 30 minute period
- Multiple conditions can be applied for a single trigger, so it is possible to get very specific about the conditions under which a survey can be presented
- Triggers can run in the background, and notify the participant when a condition is met
- New rules and content can be sent while the study is active
- PiLR Health supports pre-defined study periods in which the programmed content can change
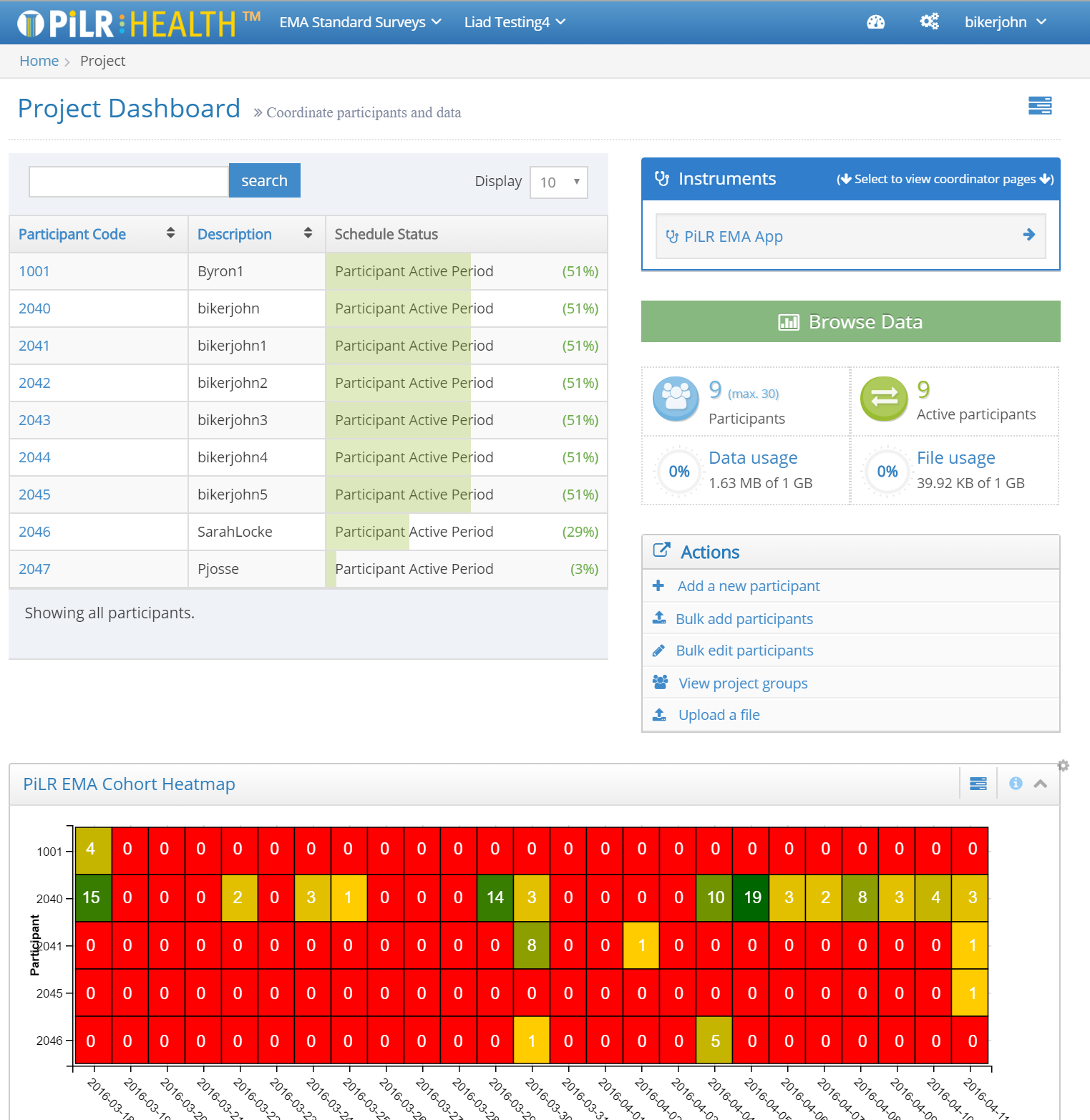
Tools for Managing Ongoing Studies
The PiLR Health Project Dashboard page makes it possible for research teams to:
- Manage study participants
- Participants can be added automatically from files
- Key participant settings (like their schedule, or the group they are assigned to) can be done in groups
- Simple dashboards make it possible to see how active participants are
- Review study data as it is collected
- If PiLR EMA is used as the mobile application, data will be forwarded to PiLR Health in real-time
- PiLR Health provides a DataViewer feature that allows the research team to select, sort, and filter data. Data can be viewed by study participant, study group, or for all participants
- The filtered view can be downloaded in several common formats (CSV, Excel, or PDF)
- The study datasets can be directly accessed from an R environment via a published data api.
